
In a nutshell
Epigenetic DNA methylation test for the detection of all carcinomas originating from the uterus, especially endometrial carcinoma, but also endocervical carcinoma.
The test is indicated in peri- and postmenopausal women with abnormal uterine bleeding.
Sample collection by cervicovaginal swab.
95% less false positives compared to transvaginal sonography.
The test reduces the number of women who have to undergo hysteroscopy with curettage by 90%.
A negative WID®-easy test result indicates a high probability that there is no malignancy, so that conservative treatment and active monitoring are an alternative to hospital referral for many patients.
Indication
Test for simple and rapid screening of women presenting with symptoms suggestive of endometrial cancer, particularly peri- and postmenopausal women with abnormal uterine bleeding.
The principle of the WID®-easy test
The procedure
A cervicovaginal swab is sent to the diagnostic laboratory.
After DNA extraction and bisulfite conversion, a quantitative PCR reaction is performed.
The WID®-easy test, which is referred to in the scientific literature as “WID-qEC test”, measures the methylation status of DNA regions of the GYPC and ZSCAN12 genes, which are known to be highly methylated in endometrial cancer and cervical cancer.
These measured values (indicated in “PMR” = “Percentage of fully Methylated Reference”) are summed to give “∑ PMR”. The result of the test depends on the determined value ∑ PMR and is provided to the physician in the test report.
Sources: www.eutops.institute and Evans et al, The Lancet Oncology, 2023
The test result*
The test result will be available to you in a few days. Please ask your laboratory for the current processing times (sample receipt in the laboratory until the report is issued) for the WID®-easy test.
The report states the ∑PMR value determined and an interpretation of the result based on Evans et al., 2023. The formulation of the test result is the responsibility of the healthcare facility that produces and uses the test.
The sampling**
Sampling is carried out using a Copan swab and medium.

Copan Swab and Medium
Sampling is carried out using the Copan FLOQswab™ (552C.80PB) and the eNAT medium (608C) from Copan, which also offers a corresponding kit (608CS01R). Both will be provided to you by your laboratory.
The procedure
Do not use any lubricant before taking the sample for the WID®-easy test.
The sample collection for the WID®-easy test takes place
- before taking a sample for another test, e.g., for a PAP smear.
- before inserting another substance into the vagina or near the cervix, such as acetic acid, for visual inspection (VIA).
- before transvaginal sonography
- or at the earliest three days after these measures.
Detailed instructions for sampling can be found under the following link.
Limitations
The WID®-easy test detects the methylation patterns of tumor DNA indicative of endometrial cancer.
Conditions that restrict the drainage of tumor DNA from the uterine cavity into the vagina, such as large endocervical polyps or fibroids, may affect the sensitivity of the test.
** The specific instructions for sampling are the responsibility of the health institution that manufactures and uses the test. These instructions will be provided by the health institution. The example presented here is a current working hypothesis. As soon as more concrete information is available, the above will be updated.
S3 guideline endometrial cancer

Diagnostic pathway for patients with postmenopausal bleeding
According to the current S3 guideline on endometrial carcinoma (AWMF register number: 032/034-OL, September 2022), transvaginal sonography as well as a cytological examination, are recommended after clinical examination.
Transvaginal sonography determines the endometrial thickness and, if the thickness is ≥ 3 mm, surgical, diagnostic procedures such as Tao Brush™, Pipelle™, hysteroscopy and curettage are recommended.
In women with peri- and postmenopausal abnormal bleeding, this threshold is reached or exceeded in over 50% of cases.
(Evans et al., The Lancet Oncology, 2023).
The performance of the WID®-easy test compared to the transvaginal sonographic examination
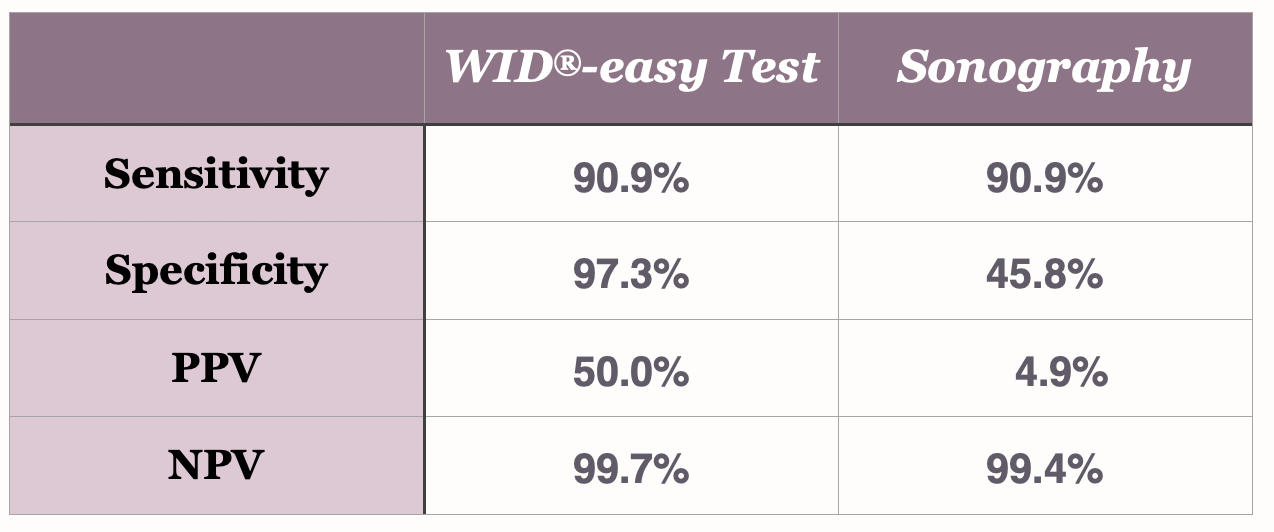
The WID®-easy test is characterized by extremely high values for sensitivity (90.9%), specificity (97.3%) and NPV (99.7%).
Above all, the PPV of the WID®-easy test is ten times higher at 50% than that of sonography, whose PPV is only 5%.
(Evans et al., The Lancet Oncology, 2023)
90 % less surgical diagnostic procedures
Over 50% false positive
on ultrasound

The gray area indicates the proportion (> 55%) of women with peri- or postmenopausal bleeding who need to undergo a surgical diagnostic procedure based on the determination of endometrial thickness by transvaginal sonography, although cancer is present in less than 3% of these women. This is a false positive rate of over 50%.
Less than 3 % false positives
with WID®-easy test

The proportion (5.2%) of women with peri- or postmenopausal bleeding who need to undergo a surgical diagnostic procedure due to a positive WID®-easy test result is shown here in gray. The proportion of false positive results is less than 3%. That’s a 95% reduction in false positives.
Extrapolated to 100 peri- and postmenopausal women presenting with abnormal bleeding, transvaginal sonography (“TVS”) will exceed the 3-mm threshold for endometrial thickness in 55 women. According to the S3 guidelines, all these women must undergo a surgical diagnostic workup (Tao Brush™, Pipelle™, hysteroscopy or curettage). More than 50 of these women will be found not to have endometrial or cervical cancer (false positive results).
Because of the higher specificity of the WID®-easy test (97.3% WID®-easy / 45.8% TVU), which results in a significantly higher positive predictive value compared with transvaginal ultrasound (PPV 50.0% WID®-easy / 4.9% TVU), the WID®-easy test suggests invasive workup in only five women. That’s a 90% reduction in invasive procedures. Half of these women will be diagnosed with cancer in the histological examination.
The false positive rate is twenty times higher with ultrasound than with the WID®-easy test (52.6% vs. 2.6%). Using the WID®-easy test can therefore avoid 95% of invasive diagnostic procedures in women who are ultimately found not to have endometrial cancer.
Sensitivity, the negative predictive value (NPV) and the rate of false negative results are comparable for both methods.
(Evans et al., The Lancet Oncology, 2023)
Robustness and complications
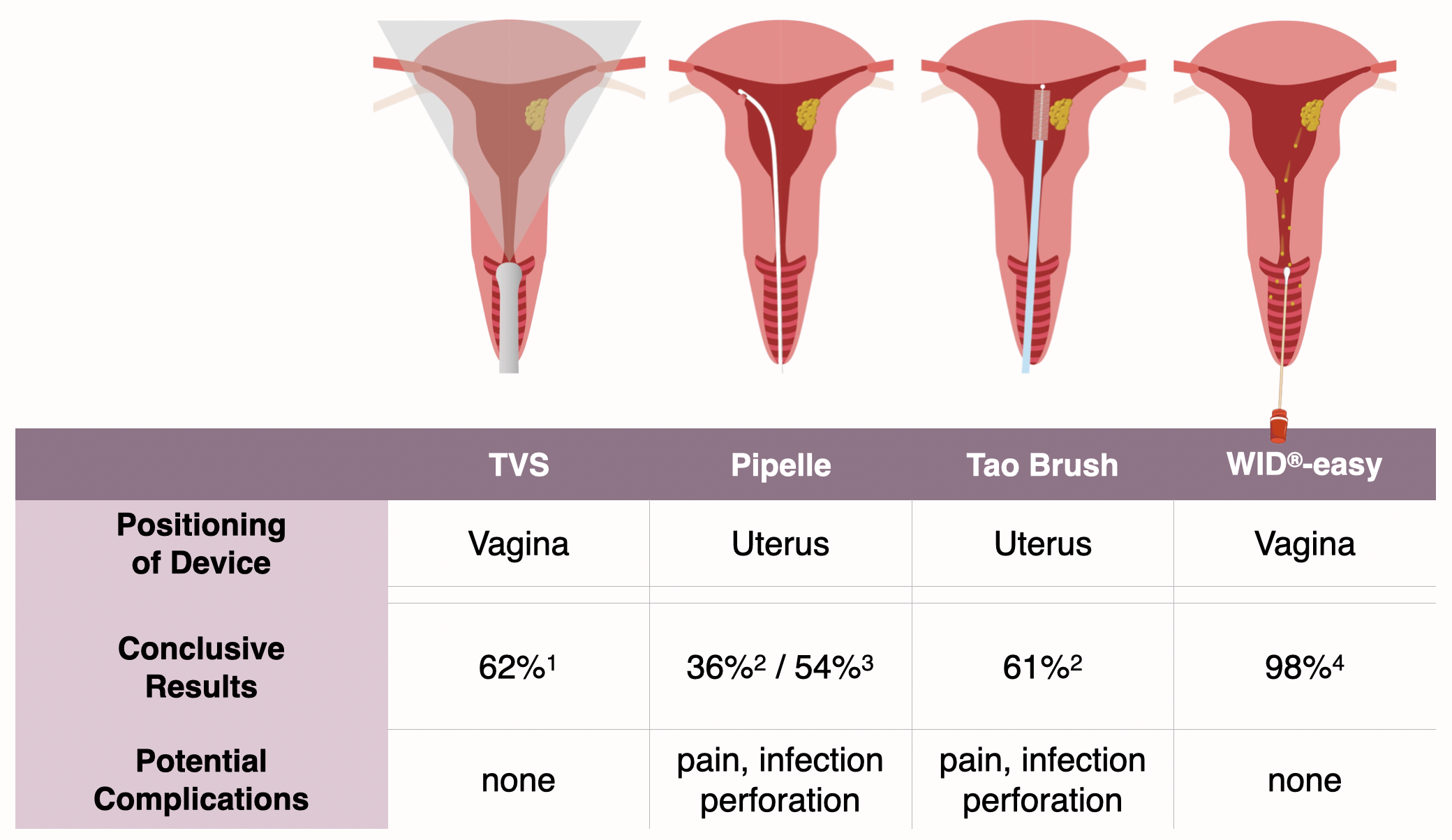
WID®-easy test compared to TVS, Pipelle™ and Tao Brush™
In contrast to examinations using transvaginal sonography, Pipelle™ or Tao Brush™, performing the WID®-easy test almost always (98%) leads to a clear result and no complications are to be expected.
In addition to the convincing performance of the WID®-easy test (sensitivity, specificity, NPV, PPV), the test is also characterized by superior robustness. Furthermore, it can be assumed that the test will not lead to any complications.
In almost 40% of cases, transvaginal sonography and the Tao Brush™ must be expected not to yield a conclusive result. In the Pipelle™ test, the expected value for the proportion of unambiguous results tends to be below 50%.
Performing the WID®-easy test, on the other hand, almost always leads to an unambiguous result (98 %).
Pipelle™ and Tao Brush™ examinations require insertion of the instruments through the cervical canal into the uterus. This can result in pain or infection, and in some cases even perforation of the uterus.
Endocervical carcinoma
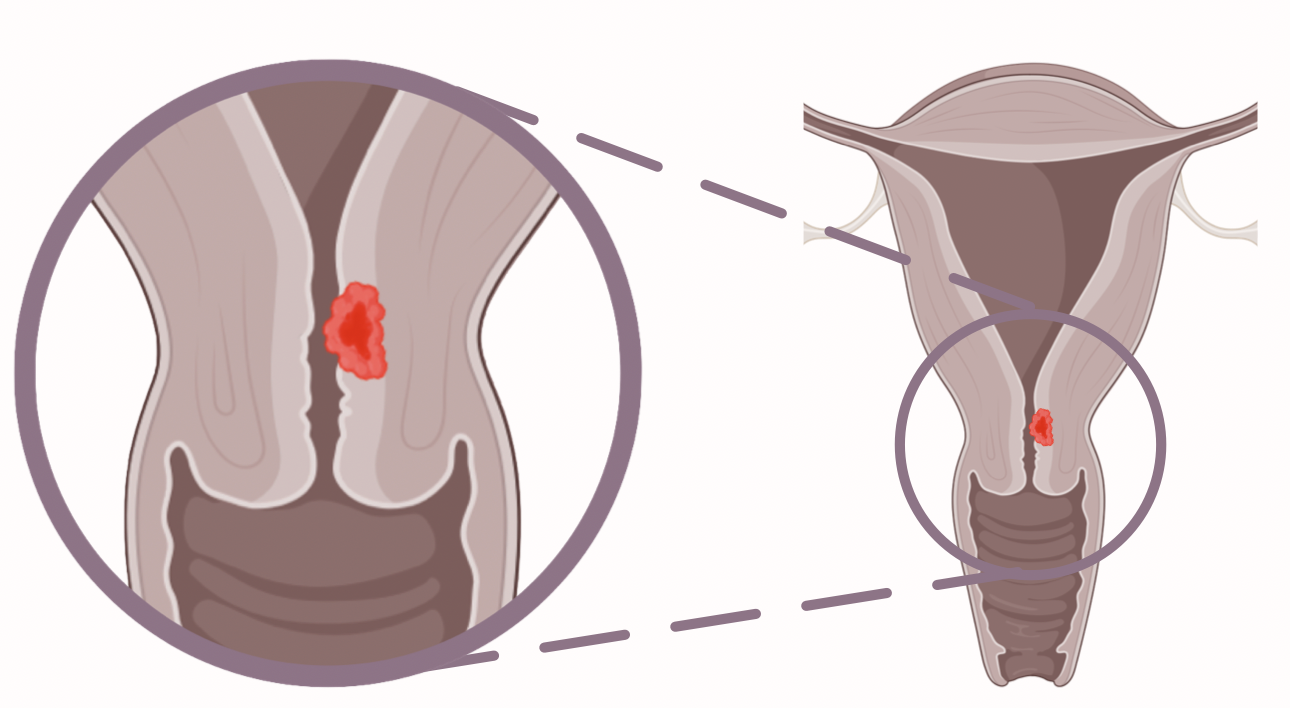
Endocervical carcinoma
Endocervical carcinomas cannot be detected during visual inspection using a speculum. Furthermore, these do not lead to an increased endometrial thickness and also remain inconspicuous in cytology.
In a case-control study, the WID®-easy test detected 21 of 22 cervical carcinomas, giving it a sensitivity of 95 %
(Schreiberhuber et al., International Journal of Cancer, 2022).
More conservative treatments
When endometrial cancer is suspected, rapid workup is critical to maximize survival if endometrial cancer is indeed present (Sud et al., The Lancet Oncology, 2020).
In case of a negative WID®-easy test, conservative treatment of women with abnormal bleeding and active monitoring with transvaginal ultrasound and the WID®-easy test is a real option.
You spare your patient a surgical intervention in the hospital and remain in close contact with your patient as her primary contact person during the conservative treatment.

Real-life comparison to the state of the art
A prospective study was conducted to compare the performance of the current improved version of the WID-qEC test with the standard procedure in the United Kingdom. The study took place at UCL-Hospital in London.
400 women aged 45 years and older with peri- or postmenopausal abnormal bleeding participated in the study.
The WID-qEC test reduces the number of women who need surgical diagnostic procedures such as hysteroscopy and curettage, even though they do not have cancer, by 87% compared to the standard procedure in the UK and by 95% compared to the standard procedure in Germany, Switzerland and Austria.
The WID-qEC test speeds up diagnosis in women with symptoms of endometrial cancer, leading to better outcomes and healthcare cost savings.
Development and validation in five different settings
This publication describes the development and validation of the WID-qEC test.
The test was developed using 726 cervicovaginal smears from women with and without endometrial cancer and validated in 562 unrelated cervicovaginal specimens using three different collection methods and two diagnostic and two predictive settings.
Real-life cohort and validation for cervical cancer.
WID-qEC test was validated in a real-life hospital cohort of 304 women.
In addition, a case-control study showed the superiority of the WID-qEC test in the detection of cervical carcinoma, especially endocervical carcinoma, compared to cytological examination.
Development of the high-throughput analytics
The article describes the technical implementation and the analytical verification of the WID-qEC test.
The described workflow includes not only the qPCR analysis itself but also the entire test process, including sample collection, automated DNA isolation, quantification and normalization and automated bisulfite conversion as well as downstream analysis and data interpretation.
This process allows laboratories to process a large number of samples exceptionally efficiently.
Comparison of different sampling methods
The study investigated whether different sampling methods influence the validity of the test. Specifically, it compared Cervex brush with PreservCyt against FLOQSwab with eNAT, physician-performed sampling with patient self-sampling, and the exact sampling site (vaginal, cervico-vaginal, or cervical) to assess their influence on the test’s performance.
The results demonstrated that the test results remained consistent irrespective of the sampling method, even for samples stored at room temperature for seven days. While self-sampling led to high specificity, the sensitivity was somewhat lower.
Overall, the WID-qEC test offers a simple, objective and non-invasive method for assessing the risk of malignancy in women with abnormal bleeding.
Are you interested in the WID®-easy test?
The WID®-easy test is manufactured and used by healthcare facilities, such as diagnostic laboratories, in accordance with the European In Vitro Diagnostic Regulation (IVDR) Article 5, paragraph 5, or corresponding national regulations.
Diagnostic laboratories that currently offer the WID®-easy test can be found on the map below. Please contact one of these laboratories directly to obtain the necessary documents and materials for commissioning, sampling and logistics.
Tyrolpath Obrist Brunhuber GmbH
Hauptplatz 4
6511 Zams, Austria
Tel: +43 5442 666 11
Mail: office@tyrolpath.at
Web: https://tyrolpath.at
labor team w ag
Blumeneggstrasse 55
9403 Goldach, Switzerland
Tel: +41 71 844 45 45
Mail: info@team-w.ch
Web: https://www.laborteam.ch

labor team w ag
Blumeneggstrasse 55, 9403 Goldach, Switzerland
Tel: +41 71 844 45 45
Mail: info@team-w.ch
Web: https://www.laborteam.ch

Tyrolpath Obrist Brunhuber GmbH
Hauptplatz 4 6511, Zams, Austria
Tel: +43 5442 666 11
Mail: office@tyrolpath.at
Web: https://tyrolpath.at




