

Features
Patient group
Peri- and postmenopausal women with abnormal bleeding.
Intended Use
Aid to diagnosis for physicians on the necessity of surgical histological clarification.
Test Principle
WID®-easy is an epigenetic PCR test determining the DNA methylation status of two regions in the GYPC and ZSCAN12 genes.
Sampling is performed by means of a cervicovaginal swab.
Performance Parameters
Relevance
Relief for the patient
The WID®-easy test reduces by 95% the number of women who need surgical diagnostic procedures such as hysteroscopy and curettage, even though they do not have cancer.
Savings for the healthcare system
WID®-easy can significantly reduce costs for the healthcare system.
In women with peri- and postmenopausal bleeding, 90% of the costs for hysteroscopies, curettage and the corresponding anesthesia can be saved.
Patient retention for the gynecologist
WID®-easy gives the office gynecologist the confidence that, in case of peri- or postmenopausal abnormal bleeding, there is no malignancy.
The physician spares the patient a surgical intervention in the hospital and remains in close contact with her as her primary contact person during conservative treatment.
Scientific Excellence
The procedure, according to the current S3 guidelines, is only necessary in case of a positive WID®-easy test. If the WID®-easy result is negative, surgery can be avoided.
According to the current S3 guidelines for diagnosis, therapy and follow-up of patients with endometrial carcinoma in Germany, Switzerland and Austria, the evaluation of peri- and postmenopausal women with abnormal bleeding is performed by determining endometrial thickness using transvaginal sonography. In the case of endometrial thickness > 3 mm, surgical histological clarification by means of uterine endoscopy (hysteroscopy) and scraping (abrasion) is indicated.
This approach leads to a high number of false positive results, resulting in many women without endometrial carcinoma needing hysteroscopy with curettage.
Use of the WID®-easy test reduces this false positive rate by approximately 95%.
Scientific evidence
The WID®-easy test is the best non-invasive test for detecting endometrial cancer and is backed by extensive scientific data.
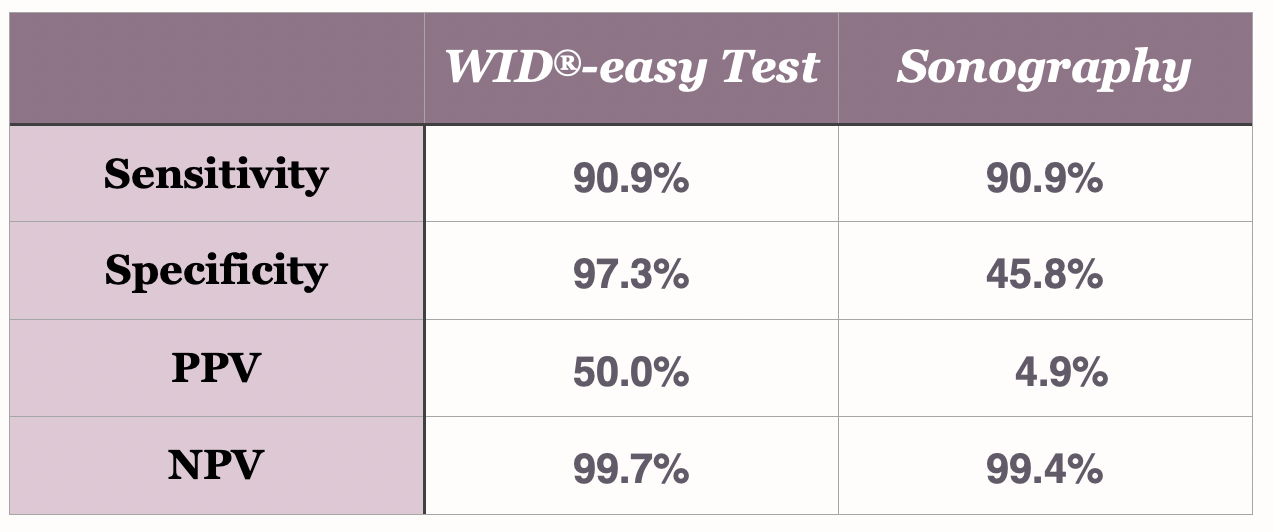
%
Sensitivity
%
Specificity
%
Positive Predictive Value (PPV)
%
Negative Predictive Value (NPV)
The direct comparison to sonography
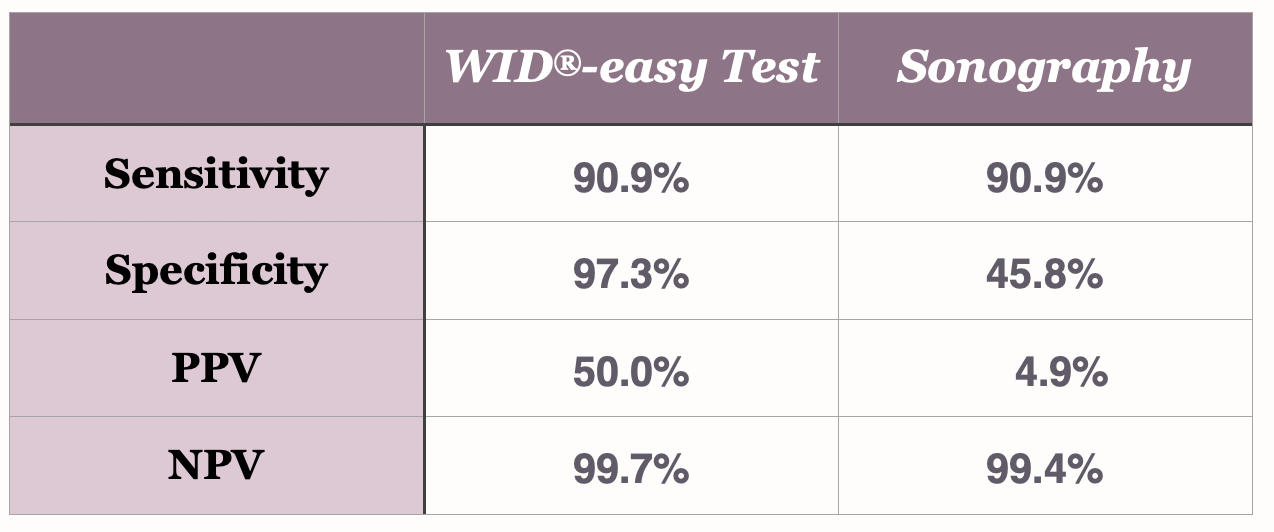
The WID®-easy test is characterized by extremely high values for sensitivity (90.9%), specificity (97.3%) and negative predictive value (99.7%). Above all, the positive predictive value (PPV) of 50% is ten times higher than that of the ultrasound examination, whose PPV is only 5%(Evans et al., The Lancet Oncology, 2023).
Real-life comparison to the state of the art
A prospective study was conducted to compare the performance of the current improved version of the WID-qEC test with the standard procedure in the United Kingdom. The study took place at UCL-Hospital in London.
400 women aged 45 years and older with peri- or postmenopausal abnormal bleeding participated in the study.
The WID-qEC test reduces the number of women who need surgical diagnostic procedures such as hysteroscopy and curettage, even though they do not have cancer, by 87% compared to the standard procedure in the UK and by 95% compared to the standard procedure in Germany, Switzerland and Austria.
The WID-qEC test speeds up diagnosis in women with symptoms of endometrial cancer, leading to better outcomes and healthcare cost savings.
Development and validation in five different settings
This publication describes the development and validation of the WID-qEC test.
The test was developed using 726 cervicovaginal smears from women with and without endometrial cancer and validated in 562 unrelated cervicovaginal specimens using three different collection methods and two diagnostic and two predictive settings.
Real-life cohort and validation for cervical cancer.
WID-qEC test was validated in a real-life hospital cohort of 304 women.
In addition, a case-control study showed the superiority of the WID-qEC test in the detection of cervical carcinoma, especially endocervical carcinoma, compared to cytological examination.
Development of the high-throughput analytics
The article describes the technical implementation and the analytical verification of the WID-qEC test.
The described workflow includes not only the qPCR analysis itself but also the entire test process, including sample collection, automated DNA isolation, quantification and normalization and automated bisulfite conversion as well as downstream analysis and data interpretation.
This process allows laboratories to process a large number of samples exceptionally efficiently.
Comparison of different sampling methods
The study investigated whether different sampling methods influence the validity of the test. Specifically, it compared Cervex brush with PreservCyt against FLOQSwab with eNAT, physician-performed sampling with patient self-sampling, and the exact sampling site (vaginal, cervico-vaginal, or cervical) to assess their influence on the test’s performance.
The results demonstrated that the test results remained consistent irrespective of the sampling method, even for samples stored at room temperature for seven days. While self-sampling led to high specificity, the sensitivity was somewhat lower.
Overall, the WID-qEC test offers a simple, objective and non-invasive method for assessing the risk of malignancy in women with abnormal bleeding.
Regulatory pathway
The WID®-easy test is manufactured and used by health institutions, such as diagnostic laboratories, following the European In Vitro Diagnostic Regulation (IVDR) Article 5, paragraph 5.
Intellectual property rights
The performance and marketing of the WID®-easy test are subject to the intellectual property rights of University College London (“UCL”) and Sola Diagnostics GmbH.
“WID” is a registered trademark of Sola Diagnostics GmbH.
The rights to perform and market the WID®-easy test can be acquired from Sola Diagnostics GmbH as a license or sublicense (in the case of UCL rights). The licensing fee is calculated based on the number of reports billed by the health institution.




