In a nutshell
- The WID®-easy test is an epigenetic test for the early detection of uterine cancer for women who suffer from abnormal uterine bleeding during or after menopause.
- The test saves many women with abnormal bleeding from having to undergo surgery to diagnose whether cancer is the cause of the bleeding.
- The test only requires a swab from the vagina.
Note:
All scientific and medical information on this page is taken from publicly available sources indicated in the text. Sola Diagnostics does not provide medical advice and makes no recommendations for action.
Uterine cancer
Uterine cancer
Uterine cancer is the most common cancer of the female reproductive organs (www.gesund.bund.de).
It predominantly affects women after menopause; only about five percent are younger than 40. Until menopause, the lining of the uterus (endometrium) renews itself every month. Periodically, the upper layers are shed and expelled with menstruation. Changes to the uterus lining in which individual cells turn into cancer cells are more common during and after menopause.
In Western countries, uterine body cancer is twice as common as cervical cancer.
In Germany, Austria and Switzerland, almost 15,000 women are diagnosed with cancer of the uterine corpus (www.ecis.jrc.ec.europa.eu) every year.
Risk factors
A major risk factor is prolonged, elevated estrogen concentration in the female body. Women with menstrual irregularities, late menopause, childlessness, or certain hormone replacement therapies have an increased risk of developing this type of cancer.
Lifestyle diseases such as obesity, high blood pressure or type 2 diabetes mellitus can increase the risk of tumours.It is known that obesity increases oestrogen production. Whether there is a risk from phytoestrogens (estrogen-like substances in food) has not yet been clarified. However, it is certain that hormone therapy with oestrogens alone increases the risk(www.gesund.bund.de).
Symptoms
Cancer at an early stage is rarely detected during routine examinations. However, the disease becomes noticeable relatively early on through sudden, abnormal bleeding. Bleeding after the menopause, irregular bleeding and flesh-colored discharge are always suspicious and should be investigated.
Abnormal uterine bleeding during or after menopause may be an indication of uterine cancer.
Diagnostics
First, your gynecologist will ask you about your general health (medical history) and perform a gynecological exam. A sonography probe is then inserted into the vagina to assess the lining of the uterus (endometrium).
If the endometrium is thicker than expected, a hysteroscopy and biopsy of the uterus (“abrasion” or “curettage”) are performed to clarify the suspicion of uterine cancer. Biopsy procedures are surgical and are relatively complex. They are performed under general anesthesia and carry the risk of bleeding, infection, or other complications of surgical procedures, such as injury or perforation of the uterus or even anesthetic incidents.
First, the doctor looks inside the uterus with a small camera (hysteroscope). They will check for visible changes to the endometrium. Following hysteroscopy, a biopsy of the uterus lining is taken and sent to the laboratory for examination. The biopsy may be taken from an area of the uterus lining that has the characteristics of cancer (targeted biopsy) or be scraped from the top layer of the womb lining (curettage).
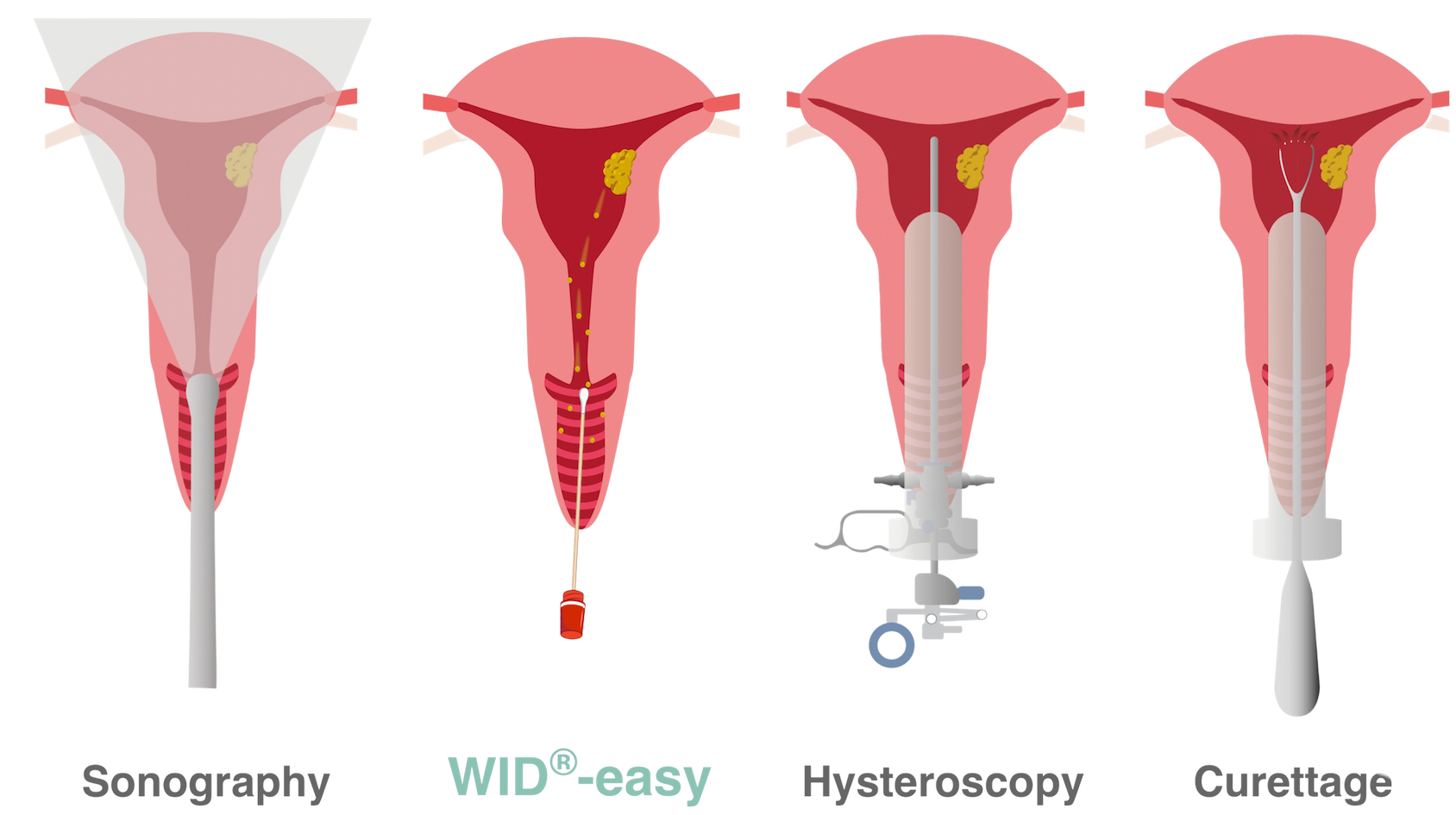
Comparison of diagnostic methods
During hysteroscopy and curettage, the cervix is surgically dilated to allow instruments to be inserted into the uterine cavity. With the WID®-easy test, on the other hand, a swab from the upper vaginal area is sufficient.
90 % less curettages

Transvaginal sonography
Out of 40 women who suffer from abnormal bleeding during or after menopause, about one woman will have uterine cancer. Nevertheless, the sonography will sound the alarm in 20 of these women (reddish background), and the women have to undergo curettage. Of these 20 women, 19 women do not have cancer.

The WID®-easy test
In the same situation, the WID®-easy test will only have a positive result in two women, so that only two women will have to undergo a curettage (highlighted in red). Of these two women, only one does not have cancer.
Compared to transvaginal sonography, significantly fewer women who do not have cancer receive a false positive test result (“false alarm”) with the WID®-easy test. As a result, significantly fewer curettages need to be performed. With sonography, there are twenty women, and with the WID®-easy test, there are only 2 women who need a curettage. That’s a 90% reduction.
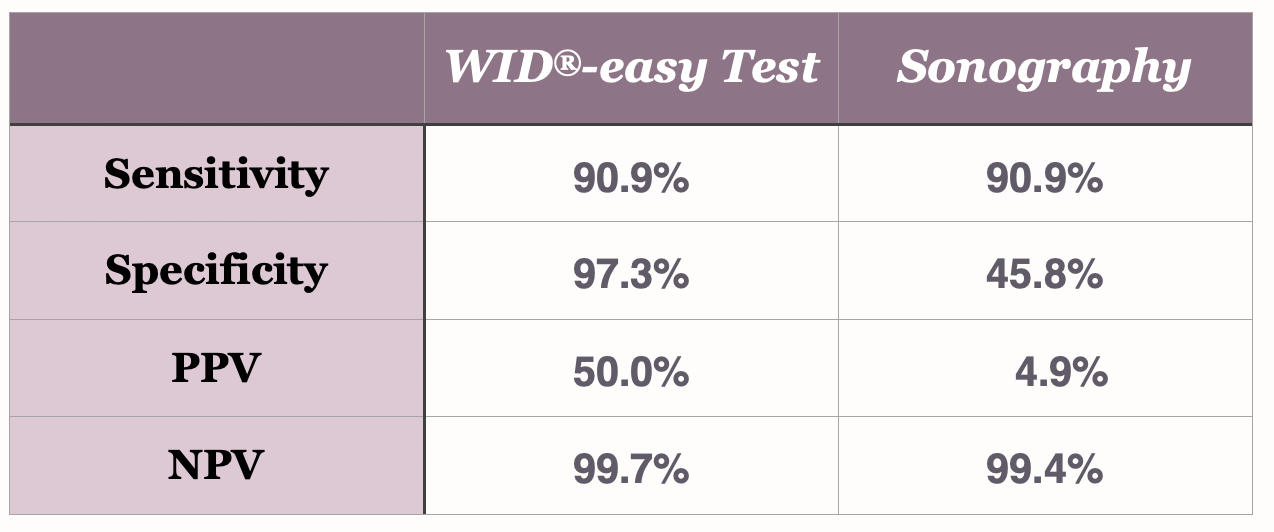
In a direct comparison between the WID®-easy test and sonography, it was shown that with the WID®-easy test, there is only one woman without cancer for every woman with cancer who has to undergo a curettage (positive predictive value [PPV] of 50%). In contrast, with sonography, there are 19 women for every woman with cancer who have a curettage, even though it later turns out that they do not have cancer (positive predictive value [PPV] of 5%). Thus, the WID®-easy test reduces the number of necessary surgical interventions in women who do not have cancer by as much as 95%.
(Source: Evans et al., The Lancet Oncology, 2023)
How is the WID®-easy test performed?
The test is very simple to perform: your doctor will take a swab from your vagina, very similar to the swab that is taken during your cervical cancer screening (“PAP smear”).
The sample is analyzed in the laboratory using PCR (similar to a Covid test) and the result is communicated to your doctor, who will discuss the result with you.
(Quelle: www.eutops.institute)
Are you interested in the WID®-easy test?
Where can you get the WID®-easy test?
Your doctor will collect the sample for the WID®-easy test. The result of the test and the conclusions and consequences to be drawn from it will be discussed with you.
If you are interested in the WID®-easy test, please discuss this with your doctor. They will be able to discuss with you whether the test is suitable and useful for you.
In which countries is the WID®-easy test available?
The WID®-easy test is already offered by diagnostic laboratories in Austria and Switzerland. Ask your doctor if they can obtain services from one of these laboratories.
Which doctors already have experience in carrying out and interpreting the WID®-easy test?
If you are interested in seeing a doctor in Switzerland, please contact Labor Team in Goldach, SG. Click here to go to the contact page of Labor Team.
If you are interested in contacting doctors in Austria or Germany, please get in touch with us. Click here to go to our contact page.
You can also find a selection of doctors who already offer the test in the following overview. You are welcome to contact the doctors directly.
Here is a selection of doctors who offer the WID®-easy test in Austria
Dr. Bernhard Bartosch
Marktgasse 2/1
1090 Vienna, Austria
Tel.: + 43 1 310 18 77
Mail: office@drbartosch.at
Web: https://www.drbartosch.at
PD Dr. Michael Hubalek
Andreas-Hofer-Straße 8
6130 Schwaz, Austria
Tel.: +43 5242 655290
Web: https://www.dr-hubalek.at/
Dr. Petra Simone Krauss
Defreggerstraße 14
6020 Innsbruck, Austria
Tel.: +43 512 365472
Mail: ordination@petrasimonekrauss.at
Web: www.petrasimonekrauss.at/ordination-innsbruck/
Dr. Monika Matal
Fasholdgasse 3/10
1130 Vienna, Austria
Tel.: +43 1 879 39 87
Mail: termin@matal.info
Web: https://www.frauenaerztin-wien.at
Dr. Sarah Oswald
Riedgasse 2, 6850 Dornbirn, Austria
Tel.: +43 5572 424 054
Mail: hallo@frauenzimmerdornbirn.at
Web: www.frauenzimmerdornbirn.at
Dr. Karin Pfau
Kirchstraße 10
6091 Goetzens, Austria
Tel.: +43 5234 21300
Mail: praxis@dr-pfau.tirol
Web: www.mein-frauenarzt.tirol
Dr. Friedrich Reh
Doeblinger Hauptstrasse 21
1190 Vienna, Austria
Tel.: +43 1 368 26 33
Mail: office@dr-reh.at
Web: www.dr-reh.at
Dr. Beate Riedmann
Riedgasse 2, 6850 Dornbirn, Austria
Tel.: +43 5572 424 054
Mail: hallo@frauenzimmerdornbirn.at
Web: www.frauenzimmerdornbirn.at
Prof. Dr. Peter Widschwendter
State Hospital Hall
Milser Strasse 10
6060 Hall in Tirol, Austria
Tel.: +43 50 504-36307
Mail: s.posch@tirol-kliniken.at
Web: www.primar-widschwendter.at

Dr. Bernhard Bartosch
Marktgasse 2/1, 1090 Vienna, Austria
Tel.: + 43 1 310 18 77
Mail: office@drbartosch.at
Web: www.drbartosch.at

PD Dr. Michael Hubalek
Andreas-Hofer-Strasse 8, 6130 Schwaz, Austria
Tel.: +43 5242 655290
Web: www.dr-hubalek.at

Dr. Petra Simone Krauss
Defreggerstrasse 14, 6020 Innsbruck, Austria
Tel.: +43 512 365472
Mail: ordination@petrasimonekrauss.at
Web: www.petrasimonekrauss.at

Dr. Monika Matal
Fasholdgasse 3/10, 1130 Vienna, Austria
Tel.: +43 1 879 39 87
Mail: termin@matal.info
Web: www.frauenaerztin-wien.at

Dr. Sarah Oswald
Riedgasse 2, 6850 Dornbirn, Austria
Tel.: +43 5572 424 054
Mail: hallo@frauenzimmerdornbirn.at
Web: www.frauenzimmerdornbirn.at

Dr. Karin Pfau
Kirchstrasse 10, 6091 Goetzens, Austria
Tel.: +43 5234 21300
Mail: praxis@dr-pfau.tirol
Web: www.mein-frauenarzt.tirol

Dr. Friedrich Reh
Doeblinger Hauptstrasse 21/7, 1190 Vienna, Austria
Tel.: +43 1 368 26 33
Mail: office@dr-reh.at
Web: www.dr-reh.at

Dr. Beate Riedmann
Riedgasse 2, 6850 Dornbirn, Austria
Tel.: +43 5572 424 054
Mail: hallo@frauenzimmerdornbirn.at
Web: www.frauenzimmerdornbirn.at

Prim. Prof. Dr. Peter Widschwendter
State Hospital Hall, Milser Strasse 10, 6060 Hall in Tirol, Austria
Tel.: +43 50 504-36307
Mail: s.posch@tirol-kliniken.at
Web: www.primar-widschwendter.at
What does the WID®-easy test cost?
Discuss the cost of the test with your doctor. The costs will include the following:
- Costs for the diagnostic laboratory to perform the test and report to your doctor. This also includes the license fee of Sola Diagnostics GmbH on a pro-rata basis.
- Your doctor’s costs for sample collection and consultation.
The costs that you as a patient have to bear also depend on the country in which you or your physician and the laboratory performing the test are located, as well as on your insurance status.
For those who want to know the details
Sensitivity, specificity and the predictive value (PPV/NPV)
The accuracy of the WID®-easy test
%
Specificity
%
Sensitivity
%
Positive Predictive Value (PPV)
%
Negative Predictive Value (NPV)
The direct comparison to sonography
The WID®-easy test is characterized by extremely high values for sensitivity (90.9%), specificity (97.3%) and negative predictive value (99.7%).
Vor allem aber ist der positive Vorhersagewert mit 50 % zehnmal höher als der der Sonographie, deren PPV nur 5 % beträgt (Evans et al., The Lancet Oncology, 2023).
How does the WID®-easy test work?
The WID®-easy test is a so-called epigenetic DNA methylation test.
DNA” is the term used to describe the human genetic material that parents pass on to their children. It consists of various long filaments (“chromosomes”) that are tightly coiled in every cell of the body. DNA plays an important role in controlling the biological processes of the cell. Different regions of the DNA have different functions.
The so-called “DNA methylation” is a process that controls the activity of the different DNA regions. You can think of methylation as little marks on DNA.
This makes it possible for different processes to take place in different cells and for the cells to take on different tasks. DNA methylation is therefore not a genetic mutation but merely a modification of the DNA.

The principle of the WID®-easy test
The WID®-easy test measures the methylation status of DNA regions known to be differentially methylated in uterine cancer.
Environmental influence on genes
Unlike the static DNA sequence, epigenetic DNA methylation patterns are subject to change and can also be influenced by the environment and personal lifestyle.
